1.12 understand that the Periodic Table as the
arrangement of elements in order of atomic
number
The number of protons in the element’s atom increases across the periodic table (the atomic number = the number of protons in an atom).
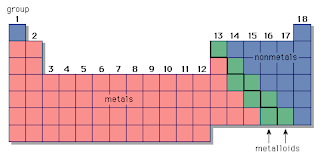
2.1 understand the terms group and period
Group: a group is a vertical column of element – the number of valence electrons (outer shell electrons) is the same as the group number of the element.
Period: a horizontal row of elements, the number of electron shells is the same as the period number of the element.
2.2 recall the positions of metals and non-metals in the Periodic Table
2.3 explain the classification of elements as metals or non-metals on the basis of their electrical conductivity and the acid-base character of their oxides
Metals are generally conductive. Non metals (excluding graphite) are not conductive. If and element is conductive and it’s oxide is basic then the element is a metal. If an element is not conductive and it’s oxide is acidic then it’s a non metal.
2.4 understand why elements in the same group of the Periodic Table have similar chemical properties
The chemical properties of elements are determined by the ease with which they lose or gain electrons. Since elements belonging to a particular group have the same number of valence electrons they show similarities in their chemical properties.
this blog is also one of the great. I am an IGCSE student and i did need this worsheet a lot.
ReplyDeleteThank you very much
Thank you so much for this blog, it has honestly helped me so much with my work
ReplyDelete